Research Article |
|
Corresponding author: Bruno Massa ( bruno.massa@unipa.it ) Academic editor: Klaus-Gerhard Heller
© 2017 Bruno Massa.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Massa B (2017) Revision of the tropical African genus Tetraconcha (Orthoptera: Tettigoniidae: Phaneropterinae) with the description of ten new species. Journal of Orthoptera Research 26(2): 211-232. https://doi.org/10.3897/jor.26.21469
|
Abstract
Only five species of the genus Tetraconcha Karsch, 1890 have been previously known; they inhabit tropical forests of central and western Africa. Generally, specimens belonging to this genus are scarcely represented in museum collections, probably due to the difficulty in finding them, but also for the fragility of their body and legs. During some recent expeditions in the Central African Republic and Ivory Coast it was possible to put together an abundant amount of specimens. This allowed the present author to revise the genus and to find valid characters to distinguish different species. On the whole, ten new species were discovered and the total number now amounts to fifteen species. Interestingly, in the Dzanga-N’Doki National Park (Central African Republic) seven sister species, previously unknown, live together with T. smaragdina; it was possible to separate them by the shape and number of teeth of the stridulatory file under the left tegmen, and later other taxonomical characters were provided. This may be considered a case of evolutionary radiation; that is, Tetraconcha species in the Dzanga-N’Doki National Park evolved traits that primarily linked to sound communication. This radiation very probably occurred randomly, possibly driven by genetic drift.
Key words
distribution, evolutionary radiation, stridulatory file, taxonomy
Introduction
According to
The availability of a long series of specimens procured in 2005–2012 by Philippe Annoyer and Philippe Moretto from the Central African Republic provided the possibility to divide them into seven taxonomic units, vaguely similar to T. smaragdina. Specimens were collected during the night, attracted to a lamp in the same site and dates within the tropical forest of the Dzanga-N’Doki National Park. During a visit to the Naturhistorisches Museum of Vienna the type of T. smaragdina, considered probably lost (
Material and methods
Series of specimens were obtained from Marios Aristophanous, Philippe Annoyer, Samuel Danflous, Philippe Moretto, Enrico Ruzzier and the present author; further specimens were examined from collections housed in the museums cited below.
Abbreviations used in this paper
BMPC Bruno Massa Collection, Palermo;
MRT Museo Regionale di Storia Naturale, Terrasini (Palermo);
MZUF Museo di Zoologia dell’Università “La Specola”, Florence;
NMW Naturhistorisches Museum Vienna;
PACT Philippe Annoyer Collection, Toulouse;
RBINS Royal Belgian Institute Natural Sciences, Bruxelles;
Some specimens were photographed with a Nikon Coolpix 4500 digital camera, mounted on a Wild M5 Stereomicroscope or Leica MZ75, and photos were integrated using the freeware CombineZP (
According to
Morphometric characters characterizing the stridulatory area of the species of the genus Tetraconcha.
| Species | Length of stridulatory file (mm) | Distance between left tegmen base and max width of lower cubital area (mm) | Size of upper and lower cubital areas (mm) |
|---|---|---|---|
| T. fenestrata | 1.8 | 5.4–5.6 | 1.1–1.2, 0.5–0.6 |
| T. ruzzieri sp. n. | 1.8 | 3.6 | 0.7, 0.2 |
| T. danflousi sp. n. | 1.0 | – | – |
| T. stichyrata | 1.7 | 7.0–9.0 | 1.0–1.9, 1.9–2.5 |
| T. banzyvilliana | 1.8 | 4.5–5.0 | 1.0–1.0 |
| T. smaragdina | 1.6 | 4.9–6.5 | 0.6–1.0, 0.6–0.9 |
| T. perezi sp. n. | 1.8 | 4.9–5.6 | 0.6–0.7, 0.6–0.7 |
| T. loubesi sp. n. | 2.5 | 3.5–5.2 | 0.6–0.8, 0.6–0.9 |
| T. morettoi sp. n. | 1.5 | 4.2–5.4 | 0.7–0.9, 0.6–0.8 |
| T. ndokiensis sp. n. | 1.4 | 4.0–6.0 | 0.5–0.9, 0.5–0.9 |
| T. annoyeri sp. n. | 1.5 | 4.0–6.0 | 0.5–0.9, 0.6–1.0 |
| T. fijalkowskii sp. n. | 1.3 | 2.9–3.8 | 0.4–0.5, 0.2–0.5 |
| T. omonomai sp. n. | 1.1 | 3.0–4.5 | 0.2–0.6, 0.2–0.5 |
| T. aristophanousi sp. n. | 1.8 | 3.9 | 0.6, 0.6 |
Results and discussion
Otiaphysini
Type genus
—Otiaphysa Karsch, 1889 (= Debrona Walker, 1870).
Material examined (other than Tetraconcha).—Stenamblyphyllum dilutum Karsch, 1896: Cameroon, Victoria (lectotype ♀) (MfN); Cameroon (1♀) (MCNM); Central African Republic, Dzanga-N’Doki National Park, Sangha 15.X.2008, P. Annoyer (1♂); Central African Republic, Dzanga-N’Doki National Park, Lac 1, 19.II.2012, 25.II.2012 (UV trap), P. Annoyer (2♂) (PACT); Central African Republic, Dzanga-N’Doki National Park, 29.II-1.III.2012 (UV trap) P. Moretto (1♂) (BMPC); Drepanophyllum marmoratumKarsch, 1890: Central African Republic, Dzanga-N’Doki National Park, N’Doki 25.I.2012, P. Moretto (1♂); N’Doki 24–25.II.2012 (UV trap), P. Moretto (1♀); N’Doki 14–15.II.2012 (UV trap), P. Moretto (1♀) (BMPC); Dzanga-N’Doki National Park, Lac 1, 19.II.2012, 25.II.2012 (UV trap), P. Annoyer (2♂, 1♀) (PACT); Gabon (1♂) (MNCN); Drepanophyllum corrosifolium Karsch, 1896: Equatorial Guinea, Fernando Poo, Musola I.1902, L. Fea (1♂) (MSNG); Debrona hebetata Karsch, 1889 (considered synonym ofDebrona cervinaWalker, 1870): Tanzania, Usambara II-III.1886 (holotype ♀) (MfN); Debrona cervinaWalker, 1870: Democratic Republic of Congo, Nguela 1899 (1♂); Tanzania, Dar El Salaam (1♂); Tanzania, Makond. Hochld. 8–11.XII.1910, H. Grote (1♂) (MfN); Kenya, Arabuko Sokoke forest 8–24.VI.1998, L. Bartolozzi and A. Sforzi (2♂, 1♀) (MZUF).
According to
The distribution of the genus Debrona covers the eastern and southern areas of Africa, from Tanzania and Kenya to South Africa. Drepanophyllum and Stenamblyphyllum species occur in central Africa, while Tetraconchais restricted to central and western Africa.
Tetraconcha
Karsch, 1890a. Entom. Nachricht. 16: 61.
Remarks
—The main characters of the genus are the following (
Species account
Tetraconcha ruzzieri sp. n.
Material examined and depository
—Ivory Coast, Aszani N. Park 26.XI–1.XII.2015, 05°14'33.7"N, 04°48'06.2"W (light trap), M. Aristophanous, P. Moretto, E. Ruzzier (♂ holotype); Ivory Coast, Taï Nat. Park, Res. Station 5–10.VII.2015, 05°49'59.8”N, 07°20'32.0”W (light trap), M. Aristophanous, P. Moretto, E. Ruzzier (2♂ paratypes) (NHM); Ivory Coast, Taï Nat. Park, Res. Station 21.III.2017 (night), S. Danflous (1♂ paratype); Ivory Coast, Taï Nat. Park, Res. Station 4.IV.2017 (light trap), P. Moretto (1♂ paratype) (BMPC).
Debrona cervina male (Kenya, Arabuko Sokoke Forest): 1. Stridulatory area; 2. Stridulatory file below the left tegmen; 3. Cerci and subgenital plate in lateral view; 4. Habitus in dorsal view; 5. Subgenital plate in dorsal view; 6. Subgenital plate in ventral view. Debrona cervina female (same locality): 7. Ovipositor in lateral view. Drepanophyllum marmoratum female (Central African Republic, N’Doki): 8. Ovipositor in lateral view. Tetraconcha sp. (probably smaragdina) female (Cameroon, Mukonje Farm): 9. Ovipositor in lateral view. Stenamblyphyllum dilutum lectotype female (Cameroon, Victoria): 10. Ovipositor in lateral view.
Tetraconcha ruzzieri sp. n. paratype male (Ivory Coast, Taï Nat. Park): 11. Stridulatory area; 12. Stridulatory file below the left tegmen; 13. Subgenital plate in ventral view; 14. Cerci in dorsal view. Tetraconcha fenestrata holotype male (Cameroon): 15. Stridulatory area of the left tegmen; 16. Stridulatory file below the left tegmen. Tetraconcha fenestratamale (Cameroon, Mukonje Farm): 17. Subgenital plate in ventral view; 18. Cerci in dorsal view. Tetraconcha danflousi sp. n. holotype male (Ivory Coast, Taï Nat. Park): 19. Stridulatory area; 20. Stridulatory file below the left tegmen and “window” of the left tegmen; 21. Subgenital plate and cerci in ventral view; 22. Cerci in dorsal view. Figs
Color
—Head and pronotum yellow-green, abdomen brown, cerci yellow, black at the tip, tegmina green-yellow, brownish in the stridulatory area, with a translucent area. Fore femora brown with 4–6 black spots, mid and hind tibiae brown. One wide black spot is visible laterally on the metanotum, below the hind wing, present only in T. danflousi sp. n. This exclusive character excludes that it is the male of T. longipes, known only from the female sex, and was also collected from the Ivory Coast, along the coast next to the border with Ghana.
Description
—Males. Head and antennae: Fastigium of vertex narrow, sulcate above, separated from fastigium of frons. Eyes rounded, well projecting. Antennae longer than body. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed on dorsal side, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 10–12 spines, fore tibiae with 4–5 spines + 1 spur on inner side and 3 small spines on anterior ventral side, 4 spines + 1 spur on outer dorsal side, mid femora armed with 5–6 spines on outer ventral side, mid tibiae with 12–13 spines on outer and inner ventral sides + 1 spur on each side, and 7 spines + 1 spur on inner dorsal side, hind femora armed with 3–4 small spines on outer side, hind tibiae with many spines on ventral and dorsal sides + 3 spurs on each side. Thorax: Pronotum narrowing anteriorly, flat above, anterior margin incurved, posterior margin rounded, humeral sinus well developed, lobes of pronotum rounded. Tegmina narrow with rounded apices, with an evident translucent area (window), laterally on the left and on the right of stridulatory areas of the left and right tegmina, respectively. Wings longer than tegmina. Stridulatory area of left and right tegmina shown in Fig.
Female. Unknown.
Measurements
—Cf. Tables
| Species | Body length | Length of tegmina | Width of tegmina | Length of hind femur |
|---|---|---|---|---|
| T. fenestrata | ♂: 16.5 (15.2–17.8); ♀: 29.1 (24.0–31.9) | ♂: 30.6 (29.8–32.3); ♀: 30.7 (28.4–31.9) | ♂: 8.2 (7.9–9.1); ♀: 10.4 (9.2–11.6) | ♂: 23.1 (22.4–25.3); ♀: 25.1 (23.3–26.9) |
| T. ruzzieri sp. n. | 18.3 (18.0–18.5) | 29.8 (27.3–32.4) | 6.0 (5.7–6.5) | 25.2 (24.9–25.5) |
| T. danflousi sp. n. | 16.6 | 30.3 | 4.6 | 27.2 |
| T. stichyrata | ♂: 16.0 (15.2–16.5); ♀: 15.5–16.8 | ♂: 22.0 (21.5–25.5); ♀: 24.2–25.5 | ♂: 5.2 (4.9–5.5); ♀: 5.0–5.5 | ♂: 18.2 (17.8–19.0); ♀: 18.1–19.0 |
| T. banzyvilliana | 18.0–20.0 | 27.0–32.0 | 4.2–5.0 | 23.4–25.5 |
| T. smaragdina | 19.1 (16.4–22.5) | 34.1 (30.0–36.7) | 5.1 (4.1–5.9) | 25.4 (23.7–26.5) |
| T. perezi sp. n. | 19.2–20.7 | 34.0–34.3 | 3.9–5.0 | 25.6–25.8 |
| T. loubesi sp. n. | 16.8 (16.0–18.0) | 31.2 (27.9–32.9) | 5.1 (4.7–5.8) | 25.2 (22.9–26.9) |
| T. morettoi sp. n. | ♂: 17.6 (16.0–19.5); ♀: 22.0 | ♂: 32.2 (29.0–34.5); ♀: 35.5 | ♂: 5.1 (4.6–5.9); ♀: 7.1 | ♂: 24.0 (20.8–26.0); ♀: 24.8 |
| T. ndokiensis sp. n. | 17.5 (14.5–20.5) | 32.0 (28.6–37.2) | 5.4 (4.5–6.5) | 23.1 (20.1–26.0) |
| T. annoyeri sp. n. | 17.5 (16.5–19.4) | 32.4 (31.6–32.7) | 5.0 (4.3–5.9) | 23.8 (22.8–24.8) |
| T. fijalkowskii sp. n. | 15.4–19.4 | 29.3–30.1 | 4.0–4.9 | 20.1–21.0 |
| T. omonomai sp. n. | 16.4 (15.5–19.4) | 30.9 (29.6–32.1) | 4.7 (4.1–5.1) | 23.6 (21.2–25.5) |
| T. aristophanousi sp. n. | ♂: 16.7 (15.6–18.0); ♀: 26.0 | ♂: 35.9 (33.5–36.9); ♀: 37.1 | ♂: 5.3 (4.9–5.8); ♀: 11.2 | ♂: 26.1 (23.8–27.5); ♀: 25.6 |
Diagnosis
— T. ruzzieri sp. n. seems to be related to T. fenestrata, but its windows (translucent areas) on the tegmina are differently placed, like all veinlets (compare Figs
Etymology
—This species is named after Enrico Ruzzier, who, together with M. Aristophanous and P. Moretto, collected many specimens of Orthoptera in the Ivory Coast by means of light traps in 2015; the material caught was sent on loan to the present author.
Tetraconcha fenestrata
Karsch, 1890a. Entom. Nachricht. 16: 62.
Type locality
—Cameroon (MfN).
Material examined.—Cameroon (♂ holotype) (MfN); Equatorial Guinea, Fernando Póo, Basile 1901 (1♂); Cameroon, Mukonje Farm, R. Rohde (1♂, 1♀) (MSNG); Equatorial Guinea, Fernando Póo (1♂); Cameroon (1♂) (MNCN); Ivory Coast, Okem (1♂); Cameroon, Mundame (1♂, 1♀) (NMW); Cameroon, Mukonje Farm, R. Rohde (7♀); Cameroon (1♂); Cameroon, Bonamo (1♂) (RBINS).
Remarks
—T. fenestrata is the type species of the genus Tetraconcha. The ratio length/width tegmina in males is between 3.5 and 3.6, in females it is between 2.6 and 3.4 (
The name fenestrata refers to the wide translucent area (from Latin fenestra = window) on the tegmina. The stridulatory area of left and right tegmina are shown in Fig.
Distribution
—T. fenestrata covers Central and West Africa (it is known from Cameroon, Ivory Coast and Equatorial Guinea).
Tetraconcha danflousi sp. n.
Material examined and depository
—Ivory Coast, Taï Nat. Park, Res. Station 11.III.2017 (light), B. Massa (1♂ holotype) (BMPC).
Color
—Head and pronotum yellow-green, abdomen yellow-brown, cerci brown, tegmina green with black spots on the anterior margin and along the diagonal veinlets. A translucent area at the base of tegmina. Like T. ruzzieri sp. n., one wide black spot is visible laterally on the metanotum, below the hind wing. This conspicuous character allows to exclude it as male of T. longipes, known only from the female sex, also collected in the Ivory Coast, along the coast next to the border with Ghana.
Description
—Male. Head and antennae: Fastigium of vertex flat and sulcate, separated from the fastigium of frons. Eyes rounded, well-projecting. Antennae longer than body. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed above, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 9 spines, fore tibiae with 7 spines + 1 spur on both ventral sides, 7 spines + 1 spur on outer dorsal side, mid femora armed with 8 spines on anterior ventral side, mid tibiae with 25–26 spines on both ventral sides + 1 spur on each side, and 9 spines + 1 spur on inner dorsal side. Hind femora unarmed, hind tibiae with many spines on both dorsal and ventral sides + 3 spurs on each side. Thorax: Pronotum narrowing anteriorly, flat above, anterior margin incurved, posterior margin rounded, humeral sinus well developed, lateral lobes rounded. Tegmina narrow with rounded apices. Wings longer than tegmina. Stridulatory area of left and right tegmina shown in Fig.
Female. Unknown.
Measurements
—Cf. Tables
Diagnosis
—T. danflousi sp. n. may be easily recognised by its translucent area at the base of tegmina, by protruding stridulatory area and a well-developed concavity between the stridulatory area and the rest of the left tegmen (Fig.
Etymology
—This species is dedicated to the French entomologist Samuel Danflous, who collected many interesting insects and spiders in the Taï Forest (Ivory Coast) and kindly helped the author during the nocturnal collecting of Orthoptera.
Distribution
—Known only from Ivory Coast (Taï Forest National Park).
Tetraconcha stichyrata
(= T. scalaris Brunner von Wattenwyl, 1891)
Karsch, 1890b. Entom. Nachricht. 16 (23): 360.
Type material
—Barombi Station (Cameroon) (MfN).
Material examined
—Cameroon, Barombi Station (♂ holotype) (MfN); Cameroon, Rohde (1♀); Gabon (1♂) (NMW); Cameroon (1♂) (MNCN); Ivory Coast, Taï Nat. Park, Research Station 20.III.2017, 05°49'59.8"N, 07°20'32.0"W (light trap), P. Annoyer (1♂); Ivory Coast, Taï Nat. Park, Research Station 20.III.2017 (night), S. Danflous (1♀); Ivory Coast, Taï Nat. Park, Res. Station 4.IV.2017 (light trap), P. Moretto (1♂) (BMPC); Ivory Coast, Taï Nat. Park, Research Station 25.III.2017 (light), P. Annoyer (1♂) (PACT); Central African Republic, Dzanga-N’Doki National Park, Sangha, camp 4, 14.III.2005, P. Annoyer (1♂); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 4.II.2012 (light trap), P. Annoyer (1♂) (PACT); Democratic Republic of Congo, Mulunau (1800 m) 24.V.1970, T. De Stefani (1♀) (MRT).
Tetraconcha stichyrata male (Ivory Coast, Taï Nat. Park): 23. Stridulatory area; 24. Stridulatory file below the left tegmen; 25. Subgenital plate in ventral view. Tetraconcha banzyvilliana male (Cameroon): 26. Stridulatory area; 27. Stridulatory file below the left tegmen. Tetraconcha perezi sp. n. holotype male (Central African Republic, N’Doki): 28. Stridulatory area; 29. Stridulatory file below the left tegmen; 30. Subgenital plate in ventral view; 31. Cerci in dorsal view. Fig.
Remarks
—When
The stridulatory area and stridulatory file of T. stichyrataare shown in Figs
Distribution.—T. stichyrata has been recorded in Cameroon and Gabon; it is here reported from Ivory Coast. Very probably its distribution covers central and western regions of tropical Africa.
Tetraconcha longipes
Bolívar I., 1893. Ann. Soc. ent. Fr. 62: 178.
Type locality
—Assinie (Ivory Coast).
Material examined
—Ivory Coast, Assinie (♀ holotype) (MNCN).
Remarks and distribution
—Only the female is known from Assinie (Ivory Coast) (
Tetraconcha banzyvilliana
Griffini, 1909. Ann. Soc. Entom. Belgique 53: 11.
Type locality
—Banzyville (Zaire = Democratic Republic of Congo) (Museum of Tervuren).
Material examined
—Tanzania, Urwald Beni IX.X.1910, Grauer (1♀) (NMW); Cameroon (1♂, 1♀) (photos in OSF). Other 6 specimens communicated: Central African Republic, Ubangi, Karawa 1939; Democratic Republic of Congo, Uélé, Lakulu 1928–32; Democratic Republic of Congo, Bambesa X-1933 (2); Democratic Republic of Congo, Kasongo; Uganda, Forêt Semliki P.N.A. (900–1200m) X/XI-1937 (S. Hanot, pers. comm.) (RMCA).
Remarks
—When
The stridulatory area is reported in Fig.
Distribution
—It is known from Tanzania, Uganda, Central African Republic, Democratic Republic of Congo and Cameroon (
Tetraconcha perezi sp. n.
Material examined and depository
—Central African Republic, N’Doki, shore of Lake 1, 21.X.2010, 02°28'51.0N 016°13'04.5E (UV trap), P. Annoyer (1♂ holotype) (BMPC); Central African Republic, N’Doki National Park, 10.X.2008 (UV trap), P. Annoyer (1♂ paratype) (PACT).
Color
—Head, antennae, pronotum and abdomen brown, face with a yellow spot, cerci yellow, tegmina with a black spot at their base, brown with yellow veinlets, bright yellow in the stridulatory area. Femora yellow-brown or green-brown (Figs
Description
—Males. Head and antennae: Fastigium of vertex tuberculated, narrow, separated from fastigium of frons, little sulcate. Eyes rounded, well projecting. Antennae longer than body, exceeding hind femora, first segment well developed, comparatively to the other species of the genus. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed dorsally, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 6–7 spines, fore tibiae with 6–7 spines + 1 spur on inner ventral side and 6 small spines on outer ventral side, 5 spines + 1 spur on outer dorsal side, mid femora armed with 6–7 spines on outer ventral margin, mid tibiae with 15–17 spines on outer and inner ventral sides + 1 spur on each side, and 3 spines + 1 spur on inner dorsal side, hind femora armed with 1–2 small spines on outer and inner ventral sides, hind tibiae with many spines on ventral and dorsal sides + 3 spurs on each side. Thorax: Pronotum narrowing anteriorly, flat above, anterior margin straight, posterior margin rounded, humeral sinus well developed, lobes of pronotum rounded. Tegmina narrow with rounded apices. Cubital area of the left tegmen narrow (Table
Female. Unknown.
Measurements
—Cf. Tables
Diagnosis
—T. perezi sp. n. is very characteristic for the narrow fastigium of the vertex, the bright yellow stridulatory area, the subgenital plate with a very small concavity, cerci stout and strongly incurved, and the uniform brown color.
Etymology
—After the entomologist Cyrille Perez, who participated to the expeditions to Dzangha-N’Doki National Park in 2010 and 2012.
Distribution
—Known only from the Dzanga-N’Doki National Park (Central African Republic).
The ‘smaragdina-group’
Generally, most specimens not belonging to any of previous species, with a pattern of T. smaragdina, having a small black spot at the base of tegmina, were identified as T. smaragdina. In fact, they actually belong to a group of species, morphologically very similar, but separate by the following characters: stridulatory file, stridulatory area, color of veinlets of tegmina, subgenital plate and cerci shape. Within the material collected by Philippe Annoyer and Philippe Moretto in the Central African Republic in 2005–2012 it was possible to identify other six undescribed species. Among the specimens collected by Philippe Annoyer, Marios Aristophanous, Samuel Danflous, Philippe Moretto, Enrico Ruzzier and myself in the Ivory Coast in 2014, 2015, 2016 and 2017 another undescribed species was discovered. Thus seven new taxa of the “smaragdina-group” are new to science.
Tetraconcha smaragdina
Brunner von Wattenwyl, 1891. Verh. der Zoologisch-Botanischen Gesellsch. Wien 41: 115, 116.
Type locality
—Cameroon (NMW).
Material examined
—Cameroon, Mus. Lubeck. (♂ holotype); Cameroon, Mundame, Rohde (1♂) (NMW); Cameroon, Lolodorf, L. Conradt (1♂) (MfN); Cameroon (1♂) (MNCN); Central African Republic, N’Doki, shore of Lake 1, UV trap 31.I-2.II.2012, 13–14.II.2012, 15–16.II.2012, 20–23.II.2012, P. Moretto (8♂); Central African Republic, surroundings of Bambio 10.XII.2008, J. Halada (1♂) (BMPC); Central African Republic, Dzanga-N’Doki National Park, Sangha, camp 3, 9.II.2005, P. Annoyer (1♂); Central African Republic, Dzanga-N’Doki National Park, Sangha, camp 2, 24.X.2008, P. Annoyer (1♂); Central African Republic, Dzanga-N’Doki National Park, N’Doki, 10.X.2008 (light trap), P. Annoyer (1♂); Central African Republic, N’Doki, shore of Lake 1, UV trap 29.I.2012, 4.II.2012, 13.II.2012, 14.II.2012, 16.II.2012, 24.II.2012, 25.II.2012, 27.II.2012, P. Annoyer (7♂); Central African Republic, N’Doki, butterfly trap 14.XI.2010, P. Annoyer (1♂); Central African Republic, N’Doki, shore of lake 7, UV trap 1.III.2012, P. Annoyer (1♂); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 3, 25.II.2012 (light trap), P. Annoyer (1♂) (PACT); Cameroon, Mukonje Farm, R. Rohde (1♀); Democratic Republic of Congo, N Mosso Norma, Las Tumba 31.VII.1938 (1♂) (RBINS).
Tetraconcha smaragdina male (Central African Republic, N’Doki): 32. Stridulatory area (the arrow shows the distance between the base of left tegmen and the maximum width of cubital areas); 33. Stridulatory area of the holotype (Cameroon); 34. Stridulatory file below the left tegmen; 35. Cerci in dorsal view; 36. Subgenital plate in ventral view of the holotype. Tetraconcha loubesi sp. n. holotype male (Central African Republic, N’Doki): 37. Stridulatory area; 38. Stridulatory file below the left tegmen; 39. Cerci in dorsal view; 40. Subgenital plate in ventral view). Tetraconcha morettoi sp. n. holotype male (Central African Republic, N’Doki): 41. Stridulatory area; 42. Stridulatory file below the left tegmen; 43. Subgenital plate in ventral view; 44. Cerci in dorsal view. Tetraconcha ndokiensis sp. n. holotype male (Central African Republic, N’Doki): 45. Stridulatory area; 46. Stridulatory file below the left tegmen; 47. Subgenital plate and cerci in dorsal view; 48. Subgenital plate and cerci in ventral view.
Remarks
—The type specimen, considered as probably lost (
Redescription
—Males. Head and antennae: Fastigium of vertex narrow, sulcate above, separated from fastigium of frons, furrowed. Eyes rounded, well projecting. Antennae longer than body. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed dorsally, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 7–10 spines, fore tibiae with 6–7 spines + 1 spur on inner side and 3–4 small spines on outer ventral side, 3 spines + 1 spur on outer dorsal side, mid femora armed with 7–8 spines on outer ventral side, mid tibiae with 12–13 spines on outer and inner ventral sides + 1 spur on each side, and 3 spines + 1 spur on inner dorsal side, hind femora armed with 2–5 small spines on the outer [
Female.
Diagnosis
—Characters of T. smaragdina are: stridulatory area of left and right tegmina and stridulatory file as in Figs
Measurements
—Cf. Tables
Distribution
—T. smaragdina is present in Cameroon, the Democratic Republic of Congo and Central African Republic; according to
Tetraconcha loubesi sp. n.
Material examined and depository
—Central African Republic, N’Doki, shore of Lake 1, 20–23.II.2012, 02°28'51.0N, 016°13'04.5E (UV trap), P. Moretto (1♂ holotype) (MSNG); same data 4–5.II.2012, 13–14.II.2012, 20–23.II.2012 (UV trap), P. Moretto (5♂ paratypes) (BMPC); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 25.XI.2010, 14.II.2012 (light trap), P. Annoyer (2♂ paratypes) (PACT).
Color
—Head and pronotum yellow-green, abdomen yellow-brown, cerci yellow, tegmina with a black spot at their base, green with black spots on back veinlets, brownish in the stridulatory area.
Description
—Males. Head and antennae: Fastigium of vertex narrow, sulcate above, separated from fastigium of frons. Eyes rounded, well projecting. Antennae longer than body. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed dorsally, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 9–10 spines, fore tibiae with 6–7 spines + 1 spur on inner side and 3–4 small spines on outer ventral side, 2 spines + 1 spur on outer dorsal side, mid femora armed with 7–8 spines on outer ventral side, mid tibiae with 15–17 spines on outer and inner ventral sides + 1 spur on each side, and 3 spines + 1 spur on inner dorsal side, hind femora armed with 5–6 small spines on outer and 1–2 on inner ventral sides, hind tibiae with many spines on ventral and dorsal sides + 3 spurs on each side. Thorax: pronotum narrowing anteriorly, flat above, anterior margin incurved, posterior margin rounded, humeral sinus well developed, lobes of pronotum rounded. Tegmina narrow with rounded apices. Wings longer than tegmina. Stridulatory area of left and right tegmina shown in Fig.
Female: unknown.
Measurements
—Cf. Tables
Diagnosis
—T. loubesi is very similar to T. smaragdina, from which it may be separated by a different stridulatory area of the left and right tegmen (compare Figs
Etymology
—This species is named after Matias Loubes, President of the Association Tout Là-Haut, responsible for the at light captures within the forest canopy during the expedition Sangha 2012 and the expedition to Taï Forest in 2017.
Distribution
—Known only from Central African Republic.
Tetraconcha morettoi sp. n.
Material examined and depository
—Central African Republic, N’Doki, shore of Lake 1, 29.II-1.III.2012, 02°28'51.0N 016°13'04.5E (UV trap), P. Moretto (1♂ holotype) (MSNG); same data 31.I-2.II.2012, 5–6.II.2012, 13–14.II.2012, 20–23.II.2012 (UV trap), P. Moretto (9♂ paratypes); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 3.III.2012 (light trap), P. Annoyer (1♀ paratype) (BMPC); Central African Republic, Dzanga-N’Doki National Park, Sangha, camp 3, 9.II.2005, P. Annoyer (1♂ paratype); Central African Republic, Dzanga-N’Doki National Park, Sangha 10.X.2008, P. Annoyer (1♂ paratype); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 12.II.2012, 19.II.2012, 3.III.2012 (light trap), P. Annoyer (4♂ paratypes); Central African Republic, Dzanga-N’Doki National Park, N’Doki, camp 1, II.2012 (light trap), P. Annoyer (1♂ paratype) (PACT).
Color
—Head and pronotum yellow-green, abdomen yellow-brown, cerci black, tegmina with a black spot at their base, green with black spots on veinlets, in most specimens the stridulatory area is brown (Fig.
Description
—Males. Head and antennae: Fastigium of vertex narrow, sulcate above, separated from fastigium of frons. Eyes rounded, well projecting. Antennae longer than body. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed on upper margin, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 10–12 spines, fore tibiae with 5 spines + 1 spur on inner side and 2 spines on outer ventral side, 2 spines + 1 spur on outer dorsal side, mid femora armed with 7–8 spines on outer ventral side, mid tibiae with 10–12 spines on outer and inner ventral sides + 1 spur on each side, and 3 spines + 1 spur on inner dorsal side, hind femora armed with 5–6 small spines on outer and 1–2 on inner ventral sides, hind tibiae with many spines on ventral and dorsal sides + 3 spurs on each side. Thorax: Pronotum narrowing anteriorly, flat above, anterior margin incurved, posterior margin rounded, humeral sinus well developed, lobes of pronotum rounded. Tegmina narrow with rounded apices. Wings longer than tegmina. Stridulatory area of left and right tegmina shown in Fig.
Female (Fig.
Measurements
—Cf. Tables
Diagnosis
—T. morettoi is characterised mainly by black cerci, its stridulatory area of left and right tegmina (Fig.
Etymology
—This species is gratefully named after the French colleague Philippe Moretto, who collected a long series of specimens of Tetraconcha and other interesting species from the Central African Republic and the Ivory Coast.
Distribution
—Known only from Central African Republic.
Tetraconcha ndokiensis sp. n.
Material examined and depository
—Central African Republic, N’Doki, shore of Lake 1, 20–23.II.2012, 02°28'51.0N 016°13'04.5E (UV trap) P. Moretto (1♂ holotype) (MSNG); Central African Republic, Dzanga-N’Doki National Park, M’boki, 2.II.2012 (light trap), P. Annoyer (1♂ paratype); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 14.II.2012, 22.II.2012 (light trap), P. Annoyer (2♂ paratypes); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 7, 3.II.2012 (light trap), P. Annoyer (1♂ paratype) (BMPC); Central African Republic, Dzanga-N’Doki National Park, Sangha platform (54m) 23.X.2008, P. Annoyer (1♂ paratype); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 14–15.II.2012, 19.II.2012, 22.II.2012 (light trap), P. Annoyer (5♂ paratypes) (PACT).
Color
—Head and pronotum yellow-green, abdomen yellow-brown, tegmina with a black spot at their base, green with yellow spots between veinlets.
Description
—Males. Head and antennae: Fastigium of vertex narrow, sulcate dorsally, separated from fastigium of frons. Eyes rounded, well projecting. Antennae long. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed on upper margin, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 6–7 spines, fore tibiae with 4–5 spines + 1 spur on inner side and 3 spines on outer ventral side, 3 spines + 1 spur on outer dorsal side, mid femora armed with 6–7 spines on outer ventral side, mid tibiae with 15–16 spines on outer and inner ventral sides + 1 spur on each side, and 4 spines + 1 spur on inner dorsal side, hind femora armed with 6–7 small spines on outer and 1–2 on inner ventral sides, hind tibiae with many spines on ventral and dorsal sides + 3 spurs on each side. Thorax: Pronotum narrowing anteriorly, flat above, anterior margin incurved, posterior margin rounded, humeral sinus well developed, lobes of pronotum rounded. Tegmina narrow with rounded apices. Wings longer than tegmina. Stridulatory area of left and right tegmina shown in Fig.
Female. Unknown.
Measurements
—Cf. Tables
Diagnosis
—T. ndokiensis is characterised mainly by the presence of yellow spots between veinlets of tegmina (Fig.
Etymology
—After the latinized name of N’Doki, the locality where it was collected, Dzanga-N’Doki National Park in the Central African Republic.
Distribution
—Known only from Central African Republic.
Tetraconcha annoyeri sp. n.
Material examined and depository
—Central African Republic, N’Doki, shore of Lake 1, 11–12.II.2012, 02°28'51.0N 016°13'04.5E (UV trap), P. Moretto (1♂ holotype) (MSNG); same locality 20–23.II.2012, P. Moretto (1♂ paratype); same locality 24–25.II.2012, P. Moretto (1♂ paratype); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 21.II.2012 (light trap), P. Annoyer (1♂ paratype) (BMPC); Central African Republic, Dzanga-N’Doki National Park, Sangha, camp 3, 6.II.2005, P. Annoyer (1♂ paratype); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 29.XI.2010, 11.II.2012 (light trap), P. Annoyer (2♂ paratypes); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 2, 24.II.2012 (light trap), P. Annoyer (1♂ paratype) (PACT).
Tetraconcha annoyeri sp. n. holotype male (Central African Republic, N’Doki): 49. Stridulatory area; 50. Stridulatory file below the left tegmen; 51. Cerci and subgenital plate in dorsal view; 52. Cerci and subgenital plate in ventral view. Tetraconcha fijalkowskii sp. n. holotype male (Central African Republic, N’Doki): 53. Stridulatory area; 54. Stridulatory file below the left tegmen; 55. Cerci and subgenital plate in dorsal view; 56. Cerci and subgenital plate in ventral view. Tetraconcha omonomai sp. n. holotype male (Central African Republic, N’Doki): 57. Stridulatory area (the arrow shows the cubital areas); 58. Stridulatory file below the left tegmen; 59. Cerci and subgenital plate in dorsal view; 60. Cerci and subgenital plate in ventral view. Tetraconcha aristophanousi sp. n. holotype male (Ivory Coast, Taï Nat. Park): 61. Stridulatory area; 62. Stridulatory file below the left tegmen; 63. Cerci and subgenital plate in dorsal view; 64. Cerci and subgenital plate in ventral view.
Color
—The whole body yellowish, tegmina with a black spot at the base, many yellowish or whitish spots between veinlets; black spots along the veinlets of posterior area of tegmina; ventral side of hind femora generally brownish.
Description
—Males. Head and antennae: Fastigium of vertex narrow, sulcate above, separated from fastigium of frons. Eyes rounded, well projecting. Antennae long. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed dorsally, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 7–8 spines, fore tibiae with 5–6 spines + 1 spur on inner and on outer ventral sides, 3 spines + 1 spur on outer dorsal side, mid femora armed with 8–9 spines on outer ventral side, mid tibiae with 15–16 spines on outer and inner ventral sides + 1 spur on each side, and 4–5 spines + 1 spur on inner dorsal side, hind femora armed with 6 small spines on outer and 3 on inner ventral sides, hind tibiae with many spines on ventral and dorsal sides + 3 spurs on each side. Thorax: Pronotum narrowing anteriorly, flat above, anterior margin incurved, posterior margin rounded, humeral sinus well developed, lobes of pronotum rounded. Tegmina narrow with rounded apices. Wings longer than tegmina. Stridulatory area of left and right tegmina shown in Fig.
Female. Unknown.
Measurements
—Cf. Tables
Diagnosis
—T. annoyerisp. n. is characterised mainly by the presence of whitish-yellow spots on tegmina (Fig.
Etymology
—This species is named after Philippe Annoyer, President of the Association Insectes du Monde and organizer of the expedition Sangha 2012; he also collected many interesting Orthoptera during the expedition to the Taï Forest (Ivory Coast) in 2017.
Distribution
—Known only from Central African Republic.
Tetraconcha fijalkowskii sp. n.
Material examined and depository
—Central African Republic, N’Doki, shore of Lake 1, 20–23.II.2012, 02°28'51.0N 016°13'04.5E (UV trap), P. Moretto (1♂ holotype) (BMPC); Central African Republic, Dzanga-N’Doki National Park, Boda-N’Gotto, 20.I.2005 (light trap), P. Annoyer (1♂ paratype) (PACT).
Color
—Head and pronotum yellow-green, abdomen yellow-brown, tegmina with a black spot at their base, green with yellow spots between veinlets.
Description
—Males. Head and antennae: Fastigium of vertex narrow, sulcate above, separated from fastigium of frons. Eyes rounded, well projecting. Antennae long. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed dorsally, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 8–9 spines, fore tibiae with 4–5 spines + 1 spur on inner and on outer ventral sides, 2–3 spines + 1 spur on outer dorsal side, mid femora armed with 5 spines on outer ventral side, mid tibiae with 13 spines on outer and inner ventral sides + 1 spur on each side, and 4 spines + 1 spur on inner dorsal side, hind femora armed with 5 small spines on outer ventral side, hind tibiae with many spines on ventral and dorsal sides + 3 spurs on each side. Thorax: Pronotum narrowing anteriorly, flat above, anterior margin incurved, posterior margin rounded, humeral sinus well developed, lobes of pronotum rounded. Tegmina narrow with rounded apices. Wings longer than tegmina. Stridulatory area of left and right tegmina shown in Fig.
Female. Unknown.
Measurements
—Cf. Tables
Diagnosis
—T. fijalkowskiisp. n. is characterised mainly by the presence of yellow spots on tegmina (Fig.
Etymology
—T. fijalkowskii sp. n. is named after Jean-Louis Fijalkowski, logistics helper in Bangui during the preparation of the expedition Sangha 2012.
Distribution
—Known only from Central African Republic.
Tetraconcha omonomai sp. n.
Material examined and depository
—Central African Republic, N’Doki, shore of Lake 1, 13–14.II.2012, 02°28'51.0N 016°13'04.5E (UV trap), P. Moretto (1♂ holotype) (MSNG); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 15.II.2012 (light trap), P. Annoyer (1♂ paratype); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 24.II.2012 (light trap), P. Annoyer (1♂ paratype) (BMPC); Central African Republic, Dzanga-N’Doki National Park, Sangha National Park, camp 3, 5.II.2005, P. Annoyer (1♂ paratype); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 19.II.2012 (light trap), P. Annoyer (1♂ paratype); Central African Republic, Dzanga-N’Doki National Park, N’Doki, Lake 1, 1.II.2012, 16.II.2012 (light trap), P. Annoyer (2♂ paratypes) (PACT).
Color
—Head and pronotum yellow-green, abdomen yellow-brown, tegmina with a black spot at their base, green.
Description
—Males. Head and antennae: Fastigium of vertex narrow, sulcate above, separated from fastigium of frons. Eyes rounded, well projecting. Antennae long. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed dorsally, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 4–5 spines, fore tibiae with 4–5 spines + 1 spur on inner and on outer ventral sides, 3 spines + 1 spur on outer dorsal side, mid femora armed with 6–7 spines on outer ventral side, mid tibiae with 7–9 spines on outer and inner ventral sides + 1 spur on each side, and 4 spines + 1 spur on inner dorsal side, hind femora armed with 6–7 small spines on outer ventral side, hind tibiae with many spines on ventral and dorsal sides + 3 spurs on each side. Thorax: Pronotum narrowing anteriorly, flat above, anterior margin incurved, posterior margin rounded, humeral sinus well developed, lobes of pronotum rounded. Tegmina narrow with rounded apices. Compared to other species of the “smaragdina-group” cubital area between cubitus of the left tegmen narrower (Table
Female. Unknown.
Measurements
—Cf. Tables
Diagnosis
—T. omonomaisp. n. is characterised mainly by the narrow cubital area of the left tegmen, its stridulatory area of the left and right tegmina (Fig.
Etymology
—This species is named after Dieu béni Bongola Omonoma, local collector of insects within the forest during the expedition Sangha 2012.
Distribution
—Known only from Central African Republic.
Tetraconcha aristophanousi sp. n.
Material examined and depository
—Ivory Coast, Taï Nat. Park, Res. Station 5–10.VII.2015, 05°49'59.8”N, 07°20'32.0”W (light trap), M. Aristophanous, P. Moretto, E. Ruzzier (1♂ holotype, 3♂ paratypes) (NHM); Ivory Coast, Man, Mt. Tonkoui (1171m) 12–18.VII.2015, 07°26'41.9”N, 07°38'40.8”W (light trap), M. Aristophanous, P. Moretto, E. Ruzzier (1♀ paratype) (NHM); Ivory Coast, Man, Mt. Tonkoui (1200m) 29.VI-1.VII.2014, (UV trap), P. Moretto (1♂ paratype) (MSNG); Ivory Coast, Touba, Biémasso 10–11.VII.2013, 08°04'19.9”N, 07°33'05.6”W (UV trap), P. Moretto (1♂ paratype); Ivory Coast, Taï Nat. Park, Res. Station 19–20.III.2017 (light trap, platform 40 m), B. Massa (2♂ paratypes); Ivory Coast, Taï Nat. Park, Res. Station 21.III.2017 (night), S. Danflous (1♀ paratype); Ivory Coast, Taï Nat. Park, Res. Station 4.IV.2017 (light trap), P. Moretto (3♂ paratypes); Ivory Coast, Taï Nat. Park, Res. Station 2–3.IV.2017 (light trap), P. Moretto (4♂ paratypes) (BMPC); Ivory Coast, Taï Nat. Park, Res. Station 2.IV.2017 (light), P. Annoyer (1♂ paratype) (PACT); Sierra Leone, Bumbuna 6.XI.1991, W. Rossi (1♂ paratype) (coll. La Greca, MSNM).
Color
Description
—Males. Head and antennae: Fastigium of vertex narrow, sulcate above, separated from fastigium of frons. Eyes rounded, well projecting. Antennae long. Legs: Fore coxae armed with a small spine. Fore tibiae furrowed dorsally, distinctly widening above tympanum, conchate on both sides. Fore femora armed on inner ventral side with 13–14 spines, fore tibiae with 6 spines + 1 spur on inner and on outer ventral sides, 1–3 spines + 1 spur on outer dorsal side, mid femora armed with 10–12 spines on outer ventral side, mid tibiae with 14–15 spines on outer and inner ventral sides + 1 spur on each side, and 3–4 spines + 1 spur on inner dorsal side, hind femora armed with 7–8 small spines on outer and 1–2 on inner ventral sides, hind tibiae with many spines on ventral and dorsal sides + 3 spurs on each side. Thorax: Pronotum narrowing anteriorly, flat above, anterior margin incurved, posterior margin rounded, humeral sinus well developed, lobes of pronotum rounded. Tegmina narrow with rounded apices. Wings longer than tegmina. Stridulatory area of left and right tegmina shown in Fig.
Female. Two females collected in the same area with some males (Mt. Tonkoui and Taï Nat. Park, Res. Station) were available, and it is also possible to describe this sex, which resulted to be clearly different from T. longipes(compare Figs
Measurements
—Cf. Tables
Diagnosis
—T. aristophanousi is characterised by its stridulatory area, stridulatory file (Figs
Etymology
—This species is dedicated to Marios Aristophanous, who, together with P. Moretto and E. Ruzzier collected in the Ivory Coast with a light trap many specimens of Orthoptera and made them available to the author.
Distribution
—T. aristophanousi is known from Ivory Coast and Sierra Leone; it probably covers other intermediate western African countries.
Characters of females of the genus Tetraconcha (Figs 79–85 )
Females may be separated by the ratio length/width of tegmina, lying between 2.6 and 3.4 in T. fenestrata, 3.3 in T. aristophanousisp. n., 3.4 in T. longipes, between 4.0 and 4.8 in T. stichyrata, 5.0 in T. morettoi sp. n., 6.5 in T. banzyvilliana. In T. smaragdina, according to
The genus Tetraconcha is known for its high sexual dimorphism. It has not been previously mentioned that males and females differ considerably by the size of the tubercles on the frons (smaller in females) and the larger size of the mandibles in females; e.g. in T. aristophanousi sp. n. the maximum width of female mandible is 0.8, while in the male it is 0.4 mm (Fig.
The eggs of Tetraconcha are similar to those of the genus Phlaurocentrum Karsch, 1889 (
Key to males of species of the genus Tetraconcha Karsch, 1890 (cf. also Tables 1 and 2 )
T. longipes (Bolívar, 1893) is not included, being known only from the female sex)
| 1 | Tegmina with a translucent area (window) near the base. | 2 |
| – | Tegmina without a translucent area near the base. | 4 |
| 2 | Black spot laterally on the metanotum. | 3 |
| – | Metanotum without black spot, stridulatory area and stridulatory file as in Figs |
T. fenestrataKarsch, 1890 |
| 3 | Stridulatory area and stridulatory file as in Figs |
T. ruzzieri sp. n. |
| – | Stridulatory area as in Fig. |
T. danflousi sp. n. |
| 4 | Subgenital plate apically pointed, not concave (Fig. |
T. stichyrata Karsch, 1890 |
| – | Subgenital plate apically more or less concave, hind wings moderately exceeding tegmina, cubital areas less wide. | 5 |
| 5 | Tegmina brightly bicolored, stridulatory area and stridulatory file as in Figs |
T. banzyvillianaGriffini, 1909 |
| – | Tegmina uniformly colored, green, yellowish, brown. | 6 |
| 6 | Tegmina brown with bright yellow stridulatory area (Fig. |
T. perezi sp. n. |
| – | Tegmina green-yellow or green-brownish, stridulatory area not particularly bright. | 7 |
| 7 | Species green-brownish colored, often with brownish stridulatory area. Yellow or white spots on tegmina absent. | 8 |
| – | Species green-yellowish colored, small yellow or whitish spots scattered on tegmina. | 9 |
| 8 | Black spots on posterior margin of tegmina scarce or absent. Subgenital plate long and apically narrowed with a narrow concavity and two longitudinal carinae on the sides (Figs |
T. smaragdina Brunner von Wattenwyl, 1891 |
| – | 7–8 large black spots on veinlets of tegmina. Subgenital plate large and short with a fairly developed concavity (Figs |
T. morettoi sp. n. |
| 9 | Yellowish or whitish spots scattered on tegmina, ventral side of hind femora generally brownish, stridulatory area and stridulatory file as in Figs |
T. annoyeri sp. n. |
| – | Yellow spots scattered on tegmina, ventral side of hind femora not brownish. | 10 |
| 10 | Tegmina mainly green with yellow spots between veinlets. Stridulatory area, stridulatory file and subgenital plate as in Figs |
T. aristophanousi sp. n. |
| – | Tegmina green-yellowish or green-brownish with yellow spots between veinlets and small black spots on posterior margin. | 11 |
| 11 | Tegmina with many small yellow spots between veinlets, stridulatory area, stridulatory file and subgenital plate as in Figs |
T. fijalkowskii sp. n. |
| – | Tegmina with few small yellow spots between veinlets. | 12 |
| 12 | Subgenital plate long with a wide concavity (Figs |
T. omonomai sp. n. |
| – | Subgenital plate, stridulatory area and stridulatory file differently shaped. | 13 |
| 13 | Subgenital plate with a deep concavity (Figs |
T. loubesi sp. n. |
| – | Subgenital plate short with a little concavity (Figs |
T. ndokiensis sp. n. |
Habitus in lateral view of males of: 67. Tetraconcha banzyvilliana; 68. T. stichyrata; 69. T. fenestrata; 70. T. smaragdina; 71. T. ruzzieri sp. n.; 72. T. morettoi sp. n.; 73. T. perezi sp. n.; 74. T. loubesi sp. n.; 75. T. danflousi sp. n.; 76. Drepanophyllum marmoratum; 77. Stenamblyphyllum dilutum; 78. Debrona cervina.
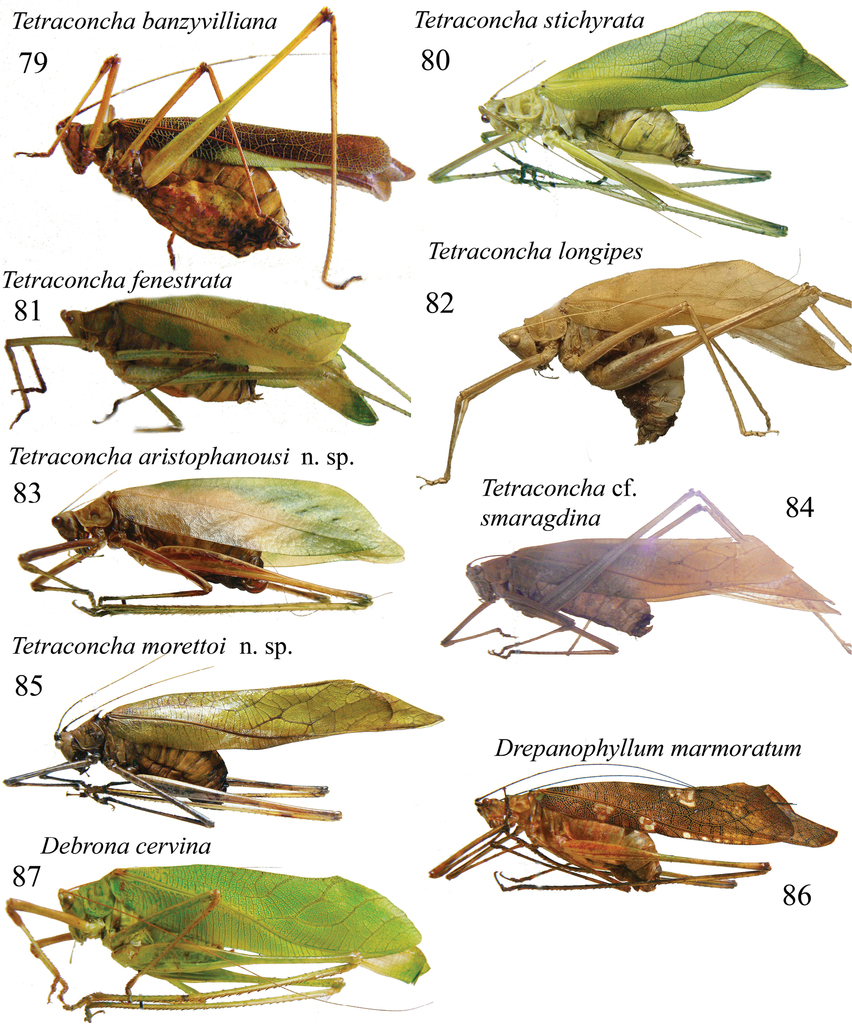
Left habitus in lateral view of females of: 79. Tetraconcha banzyvilliana; 80. T. stichyrata; 81. T. fenestrata; 82. T. longipes holotype female; 83. T. aristophanousi sp. n. paratype; 84.T. cf. smaragdina; 85. T. morettoi sp. n. Paratype; 86. Drepanophyllum marmoratum; 87. Debrona cervina.
Right habitus in lateral view of males of: 88. Tetraconcha smaragdina; 89. T. loubesi sp. n.; 90. T. morettoi sp. n.; 91. T. annoyeri sp. n.; 92. T. fijalkowskii sp. n.; 93. T. omonomai sp. n.; 94. T. ndokiensis sp. n.; 95. T. aristophanousi sp. n.; 96. T. perezi sp. n.; 97. T. danflousi sp. n.; 98. T. stichyrata; 99. T. ruzzieri sp. n..
Concluding remarks on the evolutionary radiation of Tetraconcha
Tetraconcha species are certainly not an iconic group, like Darwin’s finches of the Galápagos or the Cichlids of Lake Victoria (cf.
Concerning Tetraconcha, the increase in morphological disparity and taxonomic diversity in the ‘smaragdina group’ is very likely the effect of an evolutionary radiation, which may depend on ‘adaptive’ changes to micro-habitats within the wide tropical forest environment of central Africa. Seven species here treated were collected with the aid of a lamp in the same site and dates and certainly live close by. They may occupy different ecological niches (possibly different layers of vegetation), but live in the same forest site (Fig.
Has the emergence of many new species from a common ancestor, occurred in sympatry, accompanied by an ecological and phenotypic diversification? Phenotypic diversity of Tetraconcha species was very likely linked to ecological diversity of the African tropical forest, and potential selective pressures might have promoted the speciation through their isolation. The potential selective pressures that could have promoted such strikingly high level of speciation are unknown, and the existence of any adaptation supposedly driving the radiation has not been tested. The necessary test in this case should be to find a pattern of ecomorphological divergence demonstrating that phenotypes and ecology are closely related (cf.
According to
Thus, if we consider the ecological differences in the forest canopy of Central Africa, speciation may have occurred in Tetraconcha species both by adaptive and exaptive radiation, sensu
Finally, a climatic radiation may have co-occurred, at least for some of the species involved. Tropical forest region of Central and West Africa, also termed Guineo-Congolian region, is the second largest tropical forest of the world, with 89.3% of the total forest surface in Central and 6.0% in West Africa (
Acknowledgements
I wish to thank Philippe Moretto, who kindly let me study the material collected during the 2012 Sangha expedition (www.insectesdumonde.org), Philippe Annoyer, Président de l’Association Insectes du Monde, et Organisateur de l’expédition Sangha 2012, Matias Loubes, President of the Association Tout là-Haut, Jean-Louis Fijalkowski, for his logistic help in Bangui, the porters and guides who, from Bayanga, accompanied the expedition, the Central African population, partners and all persons who directly or indirectly supported the Sangha project team, Biodiversité en Terre Pygmée, Dieu béni Bongola Omonoma, local collector of insects within the forest during the expedition Sangha 2012, and Cyrille Perez, who participated to the expeditions Sangha in 2010 and 2012. I very much thank Philippe Annoyer, Samuel Danflous, Matias Loubes and Philippe Moretto for their collaboration and help during the collecting nights at light in the Taï National Park (Ivory Coast) in March 2017, both on the ground and at 40 m over a tree of Klainedoxia gabunensis. Philippe Annoyer very kindly provided the photographs shown in Figs
P. Moretto and I thank His Excellency Jean-Pierre Vidon, Ambassador of France in Bangui, His Excellency François Naoueyama, Minister of Environment and Ecology, His Excellency Emmanuel Bizot, Minister for Forestry, Hunting and Fishing, His Excellency the Minister for Education, Literacy, Higher Education and Research, His Excellency Karim Mekassoua, Minister of State Sangha-M’Baere; Gustave Doungoube, Environment Project Manager and Bob Konzi-Sarambo, ecology Project Manager, National Focal Point of Conservation on Biological Diversity at the Ministry of the Environment and the Ecology, for their support and assistance in obtaining necessary permits; the University of Bangui, Faculty of Sciences, Central African Republic, for the active participation in the Sangha mission, through Prof. Georgette Florence Koyt Deballé, Rector, Prof. Dr. Joachim Rouauld, Vice Rector, Prof. Dr. Jean-Laurent Syssa-Magalé, Dean of the Faculty of Sciences, University of Bangui, member of the Sangha Scientific Committee, for all resources provided to efficiently run the project; Serge Florent Bolevane Ouantinam, Research Prof., Department Head of Life Sciences, and Olga Yongo, Research Prof., for documents and granted facilitations; Jean-Bernard Yarissem, Chief Director of the WWF program in Central African Republic, Sylvain Dongolo, Angelique Todd, WWF chief scientist at Bayanga for authorizations in the Dzanga-N’Doki National Park; sampling in the canopy was made possible by the association “Tout Là-Haut” represented by Erwan Le Couillard, Matias Loubes, David Siegwalt and Yoan Ramos.
This research received support from the Synthesys Project, which is financed by European Community Research Infrastructure Action under the FP7 “Capacities” Programme at the Museo Nacional de Ciencias Naturales, Madrid (CSIC) (2013: ES-TAF-2438), the Museum für Naturkunde, Berlin (2014: DE-TAF-4109), the Naturhistorisches Museum, Vienna (2016: AT-TAF-5324), the National Museum, Prague (2016: CZ-TAF-5559) and the Royal Belgian Institute of Natural Sciences, Bruxelles (2017: BE-TAF-6319). I am especially indebted to Mercedes Paris (Museo Nacional de Ciencias Naturales of Madrid), Michael Ohl (Museum für Naturkunde of Berlin), Suzanne Randolf and Harald Bruckner (Naturhistorisches Museum, Vienna), Jérôme Constant (Royal Belgian Institute of Natural Sciences, Bruxelles), Martin Fikácek (National Museum Natural History, Prague), Stéphane Hanot (Royal Museum of Central Africa, Tervuren), Roberto Poggi, Maria Luisa Tavano and Giuliano Doria (Museo Civico di Storia Naturale ‘G. Doria’ of Genoa), Fabrizio Rigato (Museo Civico di Storia Naturale, Milan), Emanuela Palmisano (Museo Regionale di Palazzo D’Aumale, Terrasini, Palermo), Luca Bartolozzi (Museo di Zoologia ‘La Specola’, University of Florence) who facilitated the study of specimens preserved in their museums. I also very much thank Philippe Moretto, who kindly allowed me to study the material collected during 2012–2017 from central and western African countries; Philippe Annoyer, who sent me the specimens collected in the Central African Republic in 2005–2012 and in the Ivory Coast in 2017, Max Barclay, Natural History Museum, London for facilitation to study and loan for the identification of Orthoptera collected by Marios Aristophanous, Enrico Ruzzier (Natural History Museum, London) and P. Moretto in Ivory Coast. The collecting and study of the material from Ivory Coast was made possible thanks to the support of the African Natural History Research Trust (Hereford, UK) and Richard E.L. Smith. Finally I thank very much Claudia Hemp and Chunxiang Liu for the valuable comments on a first version of the manuscript, and John J. Borg for the language revision.
The collecting and study of the material from the Ivory Coast was made possible thanks to the support of the African Natural History Research Trust (Hereford, UK) and Richard E.L. Smith. Collecting authorisation were obtained as follows: 019/UB/DSV2012 of 16.I.2012 from Bangui University, Central African Republic; 135/MESRS/DGRSIT/mo of 12.VI.2015, 238/MESRS/DGRSIT/mo of 13.X.2015, 040/MESRS/DGRSIT/mo of 8.III.2016 from the Ministère de l’Einsegnement Superieur e de la Recherche Scientifique of Ivory Coast, 0429/MINEDD/OIPR/DG of 14.VII.2016, 0505/MINEDD/OIPR/DG of 18.VIII.2016 from the Ministère de l’Environnement et du Développement Durable of Ivory Coast, 021/MESRS/DGRI of 15.II.2017 from the Ministère de l’Einsegnement Superieur e de la Recherche Scientifique of Ivory Coast.
References
- Bolívar I (1893) Voyage de M. Ch. Alluaud dans le territoire d’Assinie (Afrique occidentale) en juillet et aout 1886. Orthoptères. Annales de la Société Entomologique de France 62: 169–185, pl. 1.
- Bolívar I (1906) El género "Tetraconcha" Karsch. Anales de la Sociedad Española de Historia Natural 6: 231–235.
- Brunner von Wattenwyl C (1891) Additamenta zur Monographie der Phaneropteriden. Verhandlungen der Zoologischen-Botanischen Gesellchaft in Wien 41: 1–96.
- Cigliano MM, Braun H, Eades DC, Otte D (2017) Orthoptera Species File Online. Version 2.0/4.0. http://Orthoptera.SpeciesFile.org [accessed: August 2017]
- Griffini A (1906) Ortotteri raccolti da Leonardo Fea nell’Africa occidentale. 1. Hetrodidi, Conocephalidi, Meconemidi, Pseudophyllidi, Mecopodidi e Fanerotteridi. Annali del Museo Civico di Storia Naturale di Genova 3: 358–397.
- Griffini A (1908) Phasgonuridae africane del R. Museo di Storia Naturale in Bruxelles. 6. Phaneropteridae pars 2a (reliquae species omnes). Mémoires de la Societé entomologique belgique 15: 201–226.
- Griffini A (1909) Note sopra alcune Phasgonouridae del Congo. Annales de la Société Entomologique de Belgique 53: 9–28.
- Hadley A (2008) CombineZ. Available at http://www.hadleyweb.pwp.blueyonder.co.uk [downloaded on February 2009]
- Heller K-G (2006) Song evolution and speciation in bushcrickets. In: Drosopoulos S, Claridge MF (Eds) Insect Sounds and Communication.Taylor and Francis, Boca Raton, London, New York, 137–151.
- Hemp C, Kehl S, Schultz O, Wägele JW, Hemp A (2015) Climatic fluctuations and orogenesis as motors for speciation in East Africa: case study on Parepistaurus Karsch, 1896 (Orthoptera). Systematic Entomology 40: 17–34. https://doi.org/10.1111/syen.12092
- Hemp C, Massa B (2017) Review of the African generaArantiaStål and Goetia Karsch (Orthoptera: Tettigoniidae: Phaneropterinae). Zootaxa, 4362 (4): 451–498.
- Karsch A (1890a) Orthopterologische Mitteilungen 4. Ueber Phaneropteriden. Entomologische Nachrichten 16: 57–62.
- Karsch A (1890b) Verzeichnis der von Herrn Dr. Paul Preuss auf der Barombi-Station in Deutsch-Westafrika 1890 gesammelten Locustodeen aus den Familien der Phaneropteriden, Mekonemiden und Gryllakriden. Entomologische Nachrichten 16: 353–369.
- Karsch A (1891) Übersicht der von Dr. Paul Preuss auf der Barombi-Station in Kamerun gesammelten Locustodeen. Berliner Entomologische Zeitschrift 36: 317–346.
- Kirby WF (1906) A synonymic catalogue of Orthoptera. Vol. II. OrthopteraSaltatoria. Part I (Achetidae et Phasgonuridae.). London, 562 pp.
- Leroy Y (1970) Diversités d’aspects et évolution de la dissymétrie des râpes de stridulation des insectes orthoptères Phaneropterinae. Comptes rendus hebdomadaires de l’Académie des Sciences 270: 96–99.
- Lieberman BS (2012) Adaptive radiations in the context of macroevolutionary theory: a paleontological perspective. Evolutionary Biology. Https://doi.org/10.1007/s11692-012-9165-8
- Maley J (1996) The African rain forest − main characteristics of changes in vegetation and climate from Upper Cretaceous to the Quaternary. Proceedings of the Royal Society of Edinburgh 104: 31–73. https://doi.org/10.1017/S0269727000006114
- Malhi Y, Adu-Bredu S, Asare RA, Lewis SL, Mayaux P (2013) African rainforests: past, present and future. Philosophical Transactions of the Royal Society B 368: 20120312. https://doi.org/10.1098/rstb.2012.0312
- Massa B (2013) Diversity of leaf katydids (Orthoptera: Tettigoniidae: Phaneropterinae) of Dzanga-N’Doki National Park, Central African Republic, with selected records from other African countries. Journal of Orthoptera Research 22: 125–152. https://doi.org/10.1665/034.022.0201
- Massa B (2015) Taxonomy and distribution of some katydids (OrthopteraTettigoniidae) from tropical Africa. Zookeys 524: 17–44. https://doi.org/10.3897/zookeys.524.5990
- Massa B (2016) On some interesting African katydids (OrthopteraTettigoniidae). Entomologia 4: 1–15. https://doi.org/10.4081/entomologia.2016.303
- Ragge DR (1962) A revision of the genera Drepanophyllum Karsch and Stenamblyphyllum Karsch (Orth. Tettigoniidae). Eos 38: 299–309.
- Ragge DR (1967) Contribution à la faune du Congo (Brazzaville). Mission A. Villiers et A. Descarpentries. LVI. Orthoptères, Tettigoniidae (première note). Bulletin de l’Institut Fondamental d’Afrique Noire (IFAN). Série A: Sciences Naturelles 29: 1270–1277.
- Ragge DR (1980) A review of the African Phaneropterinae with open tympana (Orthoptera: Tettigoniidae). Bulletin British Museum (Natural History) Entomology 40: 1–192.
- Schultz O, Hemp C, Hemp A, Wägele JW (2007) Molecular phylogeny of the endemic East African flightless grasshoppers Altiusambilla Jago, Usambilla (Sjöstedt) and Rhainopomma Jago (Orthoptera: Acridoidea: Lentulidae). Systematic Entomology 32: 1–8. https://doi.org/10.1111/j.1365-3113.2007.00395.x
- Simões M, Breikreuz L, Alvarado M, Baca S, Cooper JC, Heins L, Herzog K, Lieberman BS (2016) The evolving theory of evolutionary radiations. Trends in Ecology and Evolution 31: 27–34. https://doi.org/10.1016/j.tree.2015.10.007
- Sjöstedt Y (1912) Zur Orthopterenfauna des Kamerungebirges. Arkiv för Zoologi 7: 1–30. [pl. 1–3]
- Soulebeau A, Aubriot X, Gaudeul M, Rouhan G, Hennequin S, Haevermans T, Dubuisson J-Y, Jabbour F (2015) The hypothesis of adaptive radiation in evolutionary biology: hard facts about a hazy concept. Organisms, Diversity and Evolution 15: 747–761. https://doi.org/10.1007/s13127-015-0220-z
- Tortorici F, Caleca V, van Noort S, Masner L (2016) Revision of Afrotropical Dyscritobaeus Perkins, 1910 (Hymenoptera: Scelionidae). Zootaxa 4178: 1–59. https://doi.org/10.11646/zootaxa.4178.1.1
- Willis KJ, Bennett KD, Burrough SL, Macias-Fauria M, Tovar C (2013) Determining the response of African biota to climate change: using the past to model the future. Philosophical Transactions of the Royal Society B 368. https://doi.org/10.1098/rstb.2012.0491